A. ENDOSPORE STAIN
DISCUSSION
A few genera of bacteria, such as Bacillus, Clostridium and Clostridioides have the ability to produce resistant survival forms termed endospores. Unlike the reproductive spores of fungi and plants, these endospores are resistant to heat, drying, radiation, and various chemical disinfectants (see Labs 17 and 18)
- Calcium-dipicolinate, abundant
within the endospore, may stabilize and protect the endospore's DNA.
- Specialized DNA-binding proteins
saturate the endospore's DNA and protect it from heat, drying, chemicals,
and radiation.
- The cortex may osmotically remove
water from the interior of the endospore and the dehydration that results
is thought to be very important in the endospore's resistance to heat and
radiation.
- Finally, DNA repair enzymes contained within the endospore can repair damaged DNA during germination.
Endospore formation (sporulation) occurs through a complex series of events. One is produced within each vegetative bacterium. Once the endospore is formed, the vegetative portion of the bacterium is degraded and the dormant endospore is released. ( See Fig. 1.)
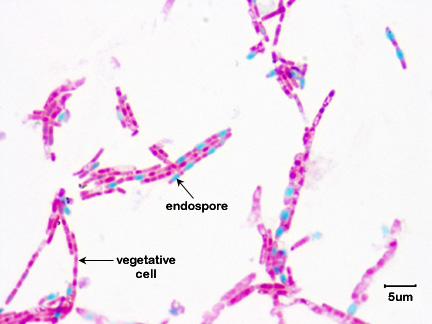
Fig. 1: Endospore stain of Bacillus megaterium
Note green endospores within pink bacilli.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
First the DNA replicates and a cytoplasmic membrane septum forms at one end of the cell. A second layer of cytoplasmic membrane then forms around one of the DNA molecules (the one that will become part of the endospore) to form a forespore. Both of these membrane layers then synthesize peptidoglycan in the space between them to form the first protective coat, the cortex. Calcium dipocolinate is also incorporated into the forming endospore. A spore coat composed of a keratin-like protein then forms around the cortex. Sometimes an outer membrane composed of lipid and protein and called an exosporium is also seen. (See Fig. 2.)
Fig. 2: Endospore Formation
A bacterial endospore within a vegetative bacterium.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Finally, the remainder of the bacterium is degraded and the endospore is released. Sporulation generally takes around 15 hours.
Click here to view an electron micrograph of an endospore of Bacillus stearothermophilus, at the Microbe Zoo web page of Michigan State University.
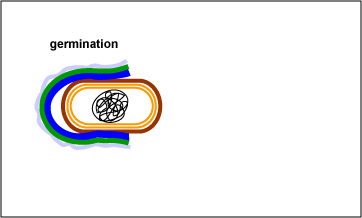
The endospore is able to survive for long periods of time until environmental conditions again become favorable for growth. The endospore then germinates, producing a single vegetative bacterium. (See Fig. 3 and Fig. 4.)
Fig. 3: Endospore Germination
Fig. 4: Germination of an endospore of Clostridium sporogenes
With the proper environmental stimuli, the endospore germinates. As the protective layers of the endospore are enzymatically broken down, a vegetative bacterium begins to form and emerge.
Note the bacillus-shaped vegetative cell emerging from the endospores.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Image provided by Kathryn Cross, Institute of Food Research.
Bacterial endospores are resistant to antibiotics, most disinfectants, and physical agents such as radiation, boiling, and drying. The impermeability of the spore coat is thought to be responsible for the endospore's resistance to chemicals. The heat resistance of endospores is due to a variety of factors:
Although harmless themselves until they germinate, bacterial endospores are involved in the transmission of some diseases to humans. Infections transmitted to humans by endospores include:
Due to the resistant nature of the endospore coats, endospores are difficult to stain. Strong dyes and vigorous staining conditions such as heat are needed. Once stained, however, endospores are equally hard to decolorize. Since few bacterial genera produce endospores, the endospore stain is a good diagnostic test for species of Bacillus and Clostridium.a. Anthrax, caused by Bacillus anthracis. (See Figs. 5A and 5B.)
Endospores can be inhaled, ingested, or enter wounds where they germinate and the vegetative bacteria subsequently replicate and produce exotoxins. In the case of the two anthrax exotoxins, two different A-components known as lethal factor (LF) and edema factor (EF) share a common B-component known as protective antigen (PA). Protective antigen, the B-component, first binds to receptors on host cells and is cleaved by a protease creating a binding site for either lethal factor or edema factor. At low levels, LF inhibits the release of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha, (TNF-alpha), and NO. This may initially reduce immune responses against the organism and its toxins. But at high levels, LF is cytolytic for macrophages, causing release of high levels of interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-alpha), and NO. Excessive release of these cytokines can lead to a massive inflammatory response and the shock cascade, similar to septic shock. Edema factor impairs phagocytosis, and inhibits production of TNF and interleukin-6 (IL-6) by monocytes. This most likely impairs host defenses.
Fig. 5A: Endospore stain of Bacillus anthracis
Fig. 5B: Scanning Electron Micrograph of the Endospores of Bacillus anthracis
Note the endospores within the streptobacillus. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Image provided by Janice Haney Carr.
Courtesy of the Centers for Disease Control and Prevention.
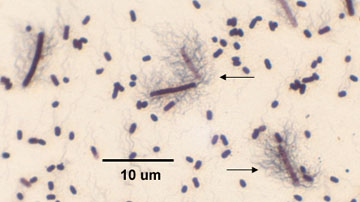
b. Tetanus, caused by Clostridium tetani. (See Fig. 6.)
Endospores enter anaerobic wounds where they germinate and the vegetative bacteria subsequently replicate and release exotoxin. Tetanus exotoxin (tetanospasmin), produced by Clostridium tetani is a neurotoxin that binds to inhibitory interneurons of the spinal cord and blocks their release of inhibitor molecules. It is these inhibitor molecules from the inhibitory interneurons that eventually allow contracted muscles to relax by stopping excitatory neurons from releasing the acetylcholine that is responsible for muscle contraction. The toxin, by blocking the release of inhibitors, keeps the involved muscles in a state of contraction and leads to spastic paralysis, a condition where opposing flexor and extensor muscles simultaneously contract. Death is usually from respiratory failure.
Fig. 6: Endospore stain of Clostridium tetani
Note the endospore within the rod gives the bacterium a "tennis racquet" shape (arrows).Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
c. Botulism, caused by Clostridium botulinum. (See Fig. 7.)
Endospores enter the anaerobic environment of improperly canned food where they germinate and subsequently replicate and at a neutral pH, secrete botulinal exotoxin. This is a neurotoxin that acts peripherally on the autonomic nervous system. For muscle stimulation, acetylcholine must be released from the neural motor end plate of the neuron at the synapse between the neuron and the muscle to be stimulated. The acetylcholine then induces contraction of the muscle fibers. The botulism exotoxin binds to and enters the presynaptic neuron and blocks its release of acetylcholine. This causes a flaccid paralysis, a weakening of the involved muscles. Death is usually from respiratory failure.
Fig. 7: Endospore stain of Clostridium botulinum
Endospores stain green while vegetative bacteria stain red. Courtesy of the Public Health Image Library (Images), Centers for Disease Control and Prevention.
d. Gas gangrene, caused by Clostridium perfringens. (See Fig. 8.)
Endospores enter anaerobic wounds where they germinate and the vegetative bacteria subsequently replicate and produce a variety of exotoxins. This bacterium produces at least 20 exotoxins that play a role in the pathogenesis of gas gangrene and producing expanding zones of dead tissue (necrosis) surrounding the bacteria. Toxins include: Alpha toxin (lecithinase) that increases the permeability of capillaries and muscle cells by breaking down lecithin in cytoplasmic membranes resulting in the gross edema associated with gas gangrene as well as being necrotizing, hemolytic, and cardiotoxic; Kappa toxin (collagenase) breaks down supportive connective tissue resulting in the mushy lesions of gas gangrene and is also necrotizing; Mu toxin (hyaluronidase) breaks down the tissue cement that holds cells together in tissue; and epsilon toxin Increases vascular permeability and causes edema and congestion in various organs including lungs and kidneys. Additional necrotizing toxins include beta toxin, iota toxin, and nu toxin. A major characteristic of gas gangrene is the ability of C. perfringens to very rapidly spread from the initial wound site, leaving behind an expanding zone of dead tissue. This organism spreads as a result of the pressure from fluid accumulation (due to increased capillary permeability from alpha toxin) and gas production (anaerobic fermentation of glucose by the organisms produces hydrogen and carbon dioxide), coupled with the breakdown of surrounding connective tissue (kappa toxin) and tissue cement (mu toxin).
Fig. 8: Endospore Stain of Clostridium perfringens
Note Gram-positive bacilli with clear endospores. > Content Providers(s): CDC/ Dr. Holderman [Public domain]
Courtesy of the Centers for Disease Control and Prevention.
e. Antibiotic-associated pseudomembranous colitis, caused by Clostridioides difficile, formerly known as Clostridium difficile. (See Fig. 9.)
Clostridioides difficile causes severe antibiotic-associated colitis and is an opportunistic Gram-positive, endospore-producing bacillus transmitted by the fecal-oral route. C. difficile is a common health-care-associated infection (HAIs) and is the most frequent cause of health-care-associated diarrhea. C. difficile infection often recurs and can progress to sepsis and death. CDC has estimated that there are about 500,000 C. difficile infections (CDI) in health-care associated patients each year and is linked to 15,000 American deaths each year. Antibiotic-associated colitis is especially common in older adults. It is thought that C. difficile survives the exposure to the antibiotic by sporulation. After the antibiotic is no longer in the body, the endospores germinate and C. difficile overgrows the intestinal tract and secretes toxin A and toxin B that have a cytotoxic effect on the epithelial cells of the colon. C. difficile has become increasingly resistant to antibiotics in recent years making treatment often difficult. There has been a great deal of success in treating the infection with fecal transplants.
Fig. 9: Scanning Electron Micrograph of Clostridioides difficile (formerly known as Clostridium difficile) from Stool
By Content Providers(s): CDC/ Lois S. Wiggs [Public domain]
Courtesy of the Centers for Disease Control and Prevention.
For further information on bacterial endospores, see the following in your CourseArc Lectures:
ORGANISMSTrypticase Soy agar plate cultures of Bacillus megaterium.
PROCEDURE (to be done individually)
1. Heat-fix a smear of Bacillus megaterium as follows:
a. Using the dropper bottle of deionized water found in your staining rack, place 1/2 of a normal sized drop of water on a clean slide by touching the dropper to the slide. Alternately, use your sterilized inoculating loop to place a drop of deionized water on the slide.
b. Using your sterile inoculating loop, aseptically remove a small amount of the culture from the edge of the growth on the agar surface and generously mix it with the drop of water until the water becomes visibly cloudy .
c. Incinerate the remaining bacteria on the inoculating loop.
d. After the inoculating loop cools, spread the suspension over approximately half of the slide to form a thin film.
e. Allow this thin suspension to completely air dry.
f. To heat-fix the bacteria to the slide, pick up the air-dried slide with coverslip forceps and hold the bottom of the slide opposite the smear against the opening of the bacto-incinerator for 10 seconds as demonstrated by your instructor. If the slide is not heated enough, all the bacteria will wash off. If it is overheated, the bacteria structural integrity can be damaged.
2. Place a piece of blotting paper over the smear and saturate with malachite green.
3. Let the malachite green sit on the slide for one minute and proceed to the next step.
4. Fill a glass beaker approximately one-fourth full with tap water, place it on a hot plate, and bring the water to a boil. Reduce the heat so the water simmers and place your slide on top of the beaker. Your slide will get hot so be sure to handle the slide with a test tube holder. Steam the slide for 5 minutes. As the malachite green evaporates, continually add more. Do not let the paper dry out!
5. After five minutes of steaming, wash the excess stain and blotting paper off the slide with water. Don't forget to wash of any dye that got onto the bottom of the slide.
6. Blot the slide dry.
7. Now flood the smear with safranin and stain for one minute.
8. Wash off the excess safranin with water, blot dry, and observe using oil immersion microscopy. With this endospore staining procedure, endospores will stain green while vegetative bacteria will stain red. (See Fig. 10.)
Fig. 10: Endospore stain of Bacillus megaterium
Note green endospores within pink bacilli. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
9. Make sure you carefully pour the used dye in your staining tray into the waste dye collection container, not down the sink.
Video review - Focusing Using Oil Immersion (1000X) Microscopy
10. Observe the demonstration slide of Bacillus anthracis. (See Fig. 11.) With this staining procedure, the vegetative bacteria stain blue and the endospores are colorless. Note the long chains of rod-shaped, endospore-containing bacteria.
Fig. 11: Endospore stain of Bacillus anthracis
Note the endospores within the streptobacillus. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
11. Observe the demonstration slide of Clostridium tetani (See Fig.12.) With this staining procedure, the vegetative bacteria stain blue and the endospores are colorless. Note the "tennis racket" appearance of the endospore-containing Clostridium.
Fig. 12: Endospore stain of Clostridium tetani
Note the endospore within the rod gives the bacterium a "tennis racquet" shape (arrows).Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
12. Endospore stain of Clostridium botulinum. (See Fig. 13.) Endospores stain green while vegetative bacteria stain red.
Fig. 13: Endospore stain of Clostridium botulinum
Endospores stain green while vegetative bacteria stain red. Courtesy of the Public Health Image Library (Images), Centers for Disease Control and Prevention.
Videos reviewing techniques used in this lab:
Video review - Focusing Using Oil Immersion (1000X) Microscopy
Video review - Aseptic Technique: Inoculation of broth tubes, slant tubes, and stab tubes
B. BACTERIAL MOTILITY
DISCUSSION
Many bacteria are capable of motility (the ability to move under their own power). Most motile bacteria propel themselves by special organelles termed flagella.
A bacterial flagellum has 3 basic parts: a filament, a hook, and a basal body.
1) The filament is the rigid, helical structure that extends from the cell surface. It is composed of the protein flagellin arranged in helical chains so as to form a hollow core. During synthesis of the flagellar filament, flagellin molecules coming off of the ribosomes are transported through the hollow core of the filament where they attach to the growing tip of the filament causing it to lengthen. With the exception of a few bacteria, such as Bdellovibrio and Vibrio cholerae, the flagellar filament is not surrounded by a sheath. (See Fig. 14.)
2) The hook is a flexible coupling between the filament and the basal body. (See Fig. 14.)
3) The basal body consists of a rod and a series of rings that anchor the flagellum to the cell wall and the cytoplasmic membrane. (See Fig. 14.) Unlike eukaryotic flagella, the bacterial flagellum has no internal fibrils and does not flex. Instead, the basal body acts as a rotary molecular motor, enabling the flagellum to rotate and propel the bacterium through the surrounding fluid. In fact, the flagellar motor rotates very rapidly.
The MotA and MotB proteins form the stator of the flagellar motor and function to generate torque for rotation of the flagellum. The MS and C rings function as the rotor. (See Fig. 14.) Energy for rotation comes from the proton motive force provided by protons moving through the Mot proteins along a concentration gradient from the peptidoglycan and periplasm towards the cytoplasm.
Fig. 14: Structure of a Bacterial Flagellum
The filament of the bacterial flagellum is connected to a hook which, in turn, is attached to a rod. The basal body of the flagellum consists of a rod and a series of rings that anchor the flagellum to the cell wall and the cytoplasmic membrane. In Gram-negative bacteria, the L ring anchors the flagellum to the lipopolysaccharide layer of the outer membrane while the P ring anchors the flagellum to the peptidoglycan portion of the cell wall. The MS ring is located in the cytoplasmic membrane and the C ring (the rotor) in the cytoplasm. The stator, composed of MotA and MotB proteins surround the MS and C rings of the motor and function to generate torque for rotation of the flagellum. Energy for rotation comes from proton motive force. Energy for rotation comes from the proton motive force provided by protons moving through the Mot proteins.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Bacterial motility constitutes unicellular behavior. In other words, motile bacteria are capable of a behavior called taxis. Taxis is a motile response to an environmental stimulus and functions to keep bacteria in an optimum environment.
The arrangement of the flagella about the bacterium is of use in classification and identification. The following flagellar arrangements may be found. (See Fig. 15.)
Fig. 15: Bacterial Flagellar Arrangements
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
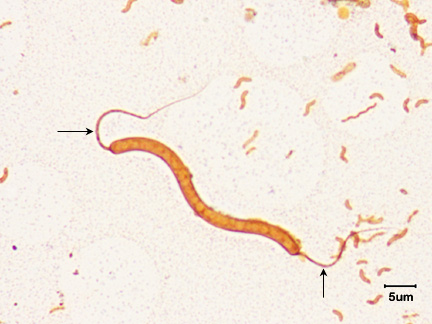
1. monotrichous - a single flagellum at one pole. (See Figs. 16A, 16B, and Fig. 16C,)
Fig. 16A: Flagella Stain Showing Monotrichous Arrangement of Flagella of a Vibrio species
Fig. 16B: Flagella Stain of Vibrio cholerae showing Monotrichous Arrangement of Flagella
Fig. 16C: Scanning Electron Micrograph Showing a Monotrichous Flagellum of Vibrio vulnificus
Note single flagellum (arrow).
Note single flagellum at each pole (arrows). Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By Content Provider: CDC [Public domain]
Courtesy of the Centers for Disease Control and Prevention.
By Content Providers(s): CDC/Dr. Edwin P. Ewing, Jr [Public domain]
Courtesy of the Centers for Disease Control and Prevention.
2. amphitrichous - a single flagellum at both poles. (See Fig. 17.)
Fig. 17: Flagella Stain of Spirillum Species Showing Amphitrichous Arrangement of Flagella
Note single flagellum at each pole (arrows). Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
3. lophotrichous - two or more flagella at one or both poles of the cell. (See Fig. 18.)
Fig. 18: Flagella Stain of Spirillum Species Showing Lophotrichous Arrangement of Flagella
Note bundle of flagella at each pole (arrows). Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
4. peritrichous - completely surrounded by flagella. (See Fig. 19.)
Fig. 19: Flagella Stain of Proteus Showing Peritrichous Arrangement of Flagella
Note the bacterium is surrounded by flagella (arrow).Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
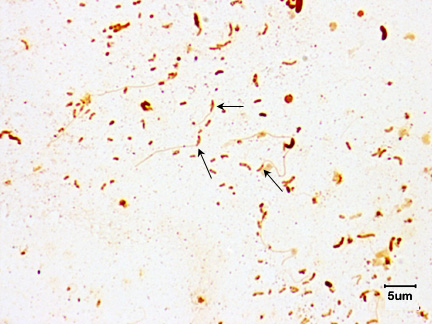
One group of bacteria, the spirochetes, has internally-located axial filaments. (see Fig. 20) or endoflagella. Axial filaments wrap around the spirochete towards the middle from both ends. They are located above the peptidoglycan cell wall but underneath the outer membrane or sheath.
Fig. 20: Spirochete Axial Filaments
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Some bacteria use motility to contact host cells and disseminate within a host. The mucosal surfaces of the respiratory tract, the intestinal tract, and the genitourinary tract constantly flush bacteria away in order to prevent colonization of host mucous membranes. Motile bacteria can use their motility and chemotaxis to swim through mucus towards mucosal epithelial cells. Many bacteria that can colonize the mucous membranes of the bladder and the intestines, in fact, are motile. Motility probably helps these bacteria move through the mucus between the mucin strands or in places where the mucus is less viscous.
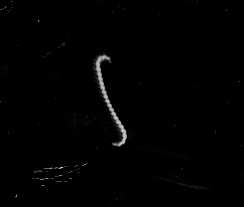
In addition, because of their thinness, their internal flagella (axial filaments), their corkscrew shape, and their motility, certain spirochetes are more readily able to penetrate host mucous membranes, skin abrasions, etc., and enter the body. Motility and penetration may also enable the spirochetes to penetrate deeper in tissue and enter the lymphatics and bloodstream and disseminate to other body sites. Spirochetes that infect humans include Treponema pallidum that causes syphilis, Leptospira that causes leptospirosis, and Borrelia burgdorferi that causes Lyme disease. (See Figs. 21A, 21B, and 21C.)
Fig. 21A: The Spirochete Treponema pallidum (arrows) in Tissue.
Fig. 21B: Darkfield Microscope Photomicrograph of the Spirochete Leptospira
Fig. 21C: Borrelia burgdorferi
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Photo Credit:Content Providers(s): CDC, Public domain, via Wikimedia Commons
For further information on bacterial flagella and motility, see the following in your CourseArc Lectures:
- Bacterial Flagella
- The Ability of Bacteria to Use Motility or Other Means to Contact Host Cells and Disseminate Within a Host
To detect bacterial motility, we can use any of the following three methods: 1) direct observation by means of special-purpose microscopes (phase-contrast and dark-field), 2) motility media, and, indirectly, 3) flagella staining.
1. Direct observation of motility using special-purpose microscopes.
Movies of bacterial motilitya. Phase-contrast microscopy
A phase-contrast microscope uses special phase-contrast objectives and a condenser assembly to control illumination and give an optical effect of direct staining. The special optics will convert slight variations in specimen thickness into corresponding visible variation in brightness. Thus, the bacterium and its structures appear darker than the background.
Phase contrast microscopy of motile Pseudomonas from YouTube.
b. Dark-field microscopy
A dark-field microscope uses a special condenser to direct light away from the objective lens. However, bacteria (or other objects) lying in the transparent medium will scatter light so that it enters the objective. This gives the optical effect of an indirect stain. The organism will appear bright against the dark background. Dark field microscopy is especially valuable in observing the very thin spirochetes. (See Fig. 21B above and Fig. 22).
Fig. 22: Darkfield Microscope Photomicrograph of the Spirochete Borrelia burgdorferi
© Jeffrey Nelson, author. Licensed for use, ASM MicrobeLibrary.
2. Motility Test medium
Semi-solid Motility Test medium may also be used to detect motility. The agar concentration (0.3%) is sufficient to form a soft gel without hindering motility. When a non-motile organism is stabbed into Motility Test medium, growth occurs only along the line of inoculation. Growth along the stab line is very sharp and defined. (See Fig. 23A.) When motile organisms are stabbed into the soft agar, they swim away from the stab line. Growth occurs throughout the tube rather than being concentrated along the line of inoculation. Growth along the stab line appears much more cloud-like as it moves away from the stab. (See Fig. 23B.) A tetrazolium salt (TTC) is incorporated into the medium. Bacterial metabolism reduces the TTC producing formazan which is red in color. The more bacteria present at any location, the darker red the growth appears.
Fig. 23A: Motility Medium Showing a Non-motile Bacterium
Fig. 23B: Motility Medium Showing a Motile Bacterium
Non-motile bacteria do not move away from the stab line. Heavy growth appears only along the stab. The dark red color indicates where the bacteria are growing.
Motile bacteria swim away from the stab line. The growth around the stab line appears more diffuse or blurry. The dark red color indicates where the bacteria are growing.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
3. Flagella staining
If we assume that bacterial flagella confer motility, flagella staining can then be used indirectly to denote bacterial motility. Since flagella are very thin (20-28 nm in diameter), they are below the resolution limits of a normal light microscope and cannot be seen unless one first treats them with special dyes and mordants that build up as layers of precipitate along the length of the flagella, making them microscopically visible. This is a delicate staining procedure and will not be attempted here. We will, however look at several demonstration flagella stains.
ORGANISMSTrypticase Soy broth cultures of Pseudomonas aeruginosa and Staphylococcus aureus. Caution: handle these organisms as pathogens.
MEDIUMMotility Test medium (2 tubes)
PROCEDURE (to be done individually and in pairs)
1. Observe the phase-contrast microscopy demonstration of motile Pseudomonas aeruginosa.
Movie of motile Pseudomonas from YouTube.
2. Observe the dark-field microscopy demonstration of motile Pseudomonas aeruginosa.
3. Take 2 tubes of Motility Test medium per pair. Stab one with Pseudomonas aeruginosa and the other with Staphylococcus aureus. Stab the bacterium about 1/2 - 3/4 of an inch into the agar, taking care not to tilt or twist the loop so that the loop comes up through the same cut as it went down. Incubate the tubes in your test tube rack at 37°C until the next lab period.
4. Observe the flagella stain demonstrations of a Vibrio species (monotrichous), Proteus vulgaris (peritrichous) and Spirillum undula (amphitrichous) as well as the dark-field photomicrograph of the spirochete Leptospira. When observing flagella stain slides, keep in mind that flagella often break off during the staining procedure so you must look carefully to observe the true flagellar arrangement.
RESULTSA. Endospore Stain
Make drawings of the various endospore stain preparations.
B. Bacterial Motility
1. Observe the phase contrast and dark-field microscopy demonstrations of bacterial motility.
2. Observe the two tubes of Motility Test medium.
Conclusion:
Conclusion:
3. Make drawings of the flagella stain demonstrations.
Flagella stain of
Proteus vulgaris
Flagella stain of
Spirillum undula
Arrangement =
Arrangement =
Arrangement =
PERFORMANCE OBJECTIVES FOR LAB 7
After completing this lab, the student will be able to perform the following objectives:
A. ENDOSPORE STAIN
DISCUSSION
1. Name two endospore-producing genera of bacteria.
2. State the function of bacterial endospores.
RESULTS
1. Recognize endospores as the "structures" observed in an endospore stain preparation.
2. Identify a bacterium as an endospore-containing Clostridium by its "tennis racket" appearance.
B. BACTERIAL MOTILITY
DISCUSSION
1. Define the following flagellar arrangements: monotrichous, lophotrichous, amphitrichous, peritrichous, and axial filaments.
2. State the function of bacterial flagella.
3. Describe three methods of testing for bacterial motility and indicate how to interpret the results.
RESULTS
1. Recognize bacterial motility when using phase-contrast or dark-field microscopy.
2. Interpret the results of Motility Test Medium.
3. Recognize monotrichous, lophotrichous, amphitrichous, and peritrichous flagellar arrangements.
SELF-QUIZ
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Last updated: March, 2023
Please send comments and inquiries to Dr. Gary Kaiser















_lores.jpg)



